Fourier-transform-infrared spectrometer (FT-IR spectrometer)
IR spectroscopy is a physical analysis method which uses infrared radiation and belongs to the molecular spectroscopy methods. Materials absorb certain frequencies when exposed to infrared radiation. The absorption bands are related to molecular energy states. IR spectrum can be visualized in a graph of infrared light absorbance vs. frequency or wavelength. The position of the absorption bands is characteristic for particular chemical bonds (⇒ functional groups) which facilitates a structural determination (⇒ identification of materials). A limitation of the method is given since the interaction between electromagnetic radiation and a molecule occurs for IR-active molecules only. During measurements the wavelength is between 7800 cm-1 and 350 cm-1 and diamond or Germanium are used as ATR (attenuated total reflection) crystal plates.

Figure: Thermofisher Scientific Nicolet iS10 FT-IR spectrometer
Chemical analysis
The following measurements are possible:
- Measurement on liquid and solid samples (⇒ studying of the surface)
- Identification of organic substances (⇒ structural determination) and aging of plastics
- Quantitative identification of known substances
- In-situ-spectroscopy (⇒ series spectrogram)
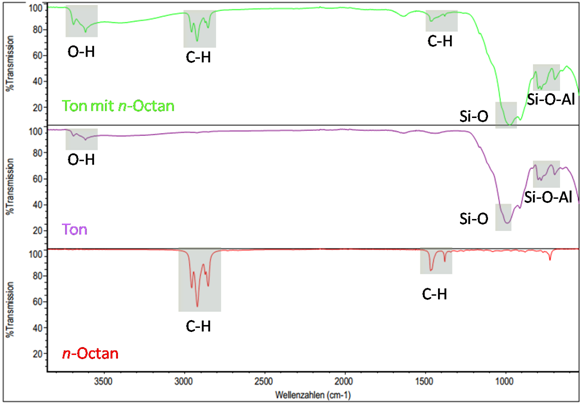
Figure: Identification of organic substances in a raw clay via IR
Gas analysis
An additional feature is the gas analysis. The gas mixture is fed into the IR- gas cell of the IR-spectrometer during measurement. An example of such transmission measurement is the coupling with the simultaneous thermal analysis (STA).